Atomic Structure Formulas
Atomic Structure is a crucial chapter when it comes to exam point of view. Keeping this in mind, we have curated the List of Atomic Structure Formulas all in one place for quick revision. Go through the detailed Formulas for Atomic Structure and have a deeper insight into related concepts. Clear all your ambiguities by making use of the Physics Formulas for Atomic Structure prevailing. Learn about Rutherford's, Thomson's Model, Bohr's Model, and many more formulas from here.
Important Atomic Structure Formulae
1. ThomsonΓÇÖs model of an atom
Entire mass and positive charge of an atom is uniformly distributed in a sphere of radius about 10-10 m and negatively charged electrons are embedded in the sphere.
2. RutherfordΓÇÖs model of an atom
Entire mass, positive charge of an atom are concentrated in a very small j region of radius about 10-15m, called nucleus. Electrons revolve round this nucleus in circular orbits.
3. Rutherford scattering, impact parameter and distance of closest approach
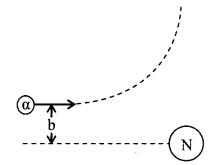
(i) The relation between the number of ╬▒-particles scattered N (╬╕) and the angle of scattering ╬╕ is
N(θ) ∝ cosec4 (θ/2)
(ii) Impact parameter
b = \(\frac{2 \mathrm{Ze}^{2} \cot (\theta / 2)}{4 \pi \varepsilon_{0} \mathrm{mv}_{0}^{2}}=\frac{\mathrm{kZe}^{2} \cot (\theta / 2)}{\mathrm{E}}\)
(iii) Distance of closest approach, If b = 0
r0 = \(\frac{2 \mathrm{Zeq}}{4 \pi \varepsilon_{0} \mathrm{mv}_{0}^{2}}=\frac{2(\mathrm{Ze})(2 \mathrm{e})}{4 \pi \varepsilon_{0} \mathrm{mv}_{0}^{2}}=\frac{\mathrm{k} 2 \mathrm{Ze}^{2}}{\mathrm{E}}\)
E = Kinetic energy of a particle
4. BohrΓÇÖs model of an atom
(i) Electrons revolve round the nucleus in circular orbits of constant energy.
Centripetal force = Coulomb force
\(\frac{m v^{2}}{r}=k \frac{Z e^{2}}{r^{2}}\)
(ii) The orbits in which the angular momentum of an electron is integral multiple h/2╧Ç, are stable orbits. (Bohr’s quantum condition)
L = mvr = \(\frac{n h}{2 \pi}\)
(iii) A photon is emitted or absorbed due to transition of an electron from one energy level to another energy level. Thus
E2 – E1 = h╬╜ (in emission)
or E1 – E2 = h╬╜ (in absorption)
(Bohr’s frequency condition)
5. State of an electron in an atom
(i) The radius of n-th orbit:
rn = \(\frac{n^{2} h^{2}}{4 \pi^{2} m K e^{2} Z}\)
= \(a_{0} \frac{n^{2}}{Z}\)
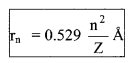
rn ∝ \(\frac{n^{2}}{Z}\)
(ii) The velocity of electron in n-th orbit:
vn = \(\frac{2 \pi \mathrm{Ke}^{2} \mathrm{Z}}{\mathrm{nh}}\)
= 2.188 x 106 \(\frac{\mathrm{Z}}{\mathrm{n}}\) m/s
= \(\frac{c}{137}\left(\frac{Z}{n}\right)\)
vn ∝ \(\frac{\mathrm{Z}}{\mathrm{n}}\)
For hydrogen
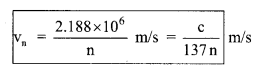
(iii) The period of an electron in n-th orbit
Tn = \(\frac{2 \pi r_{n}}{v_{n}}=\frac{n^{3} h^{3}}{4 \pi^{2} K^{2} m c^{4} Z^{2}}\) sec, Tn ∝ \(\frac{\mathrm{n}^{3}}{\mathrm{Z}^{2}}\)
(iv) Energy of an electron in n-th orbit (in hydrogen atom) For a BohrΓÇÖs atom of atomic number Z:
(a) Kinetic energy KE = \(\frac{\mathrm{KZe}^{2}}{2 \mathrm{r}_{\mathrm{n}}}=\frac{\mathrm{R} \mathrm{ch} \mathrm{Z}^{2}}{\mathrm{n}^{2}}, \mathrm{KE} \propto \frac{\mathrm{Z}^{2}}{\mathrm{n}^{2}}\)
(b) Potential energy U = \(-\frac{\mathrm{KZe}^{2}}{\mathrm{r}_{\mathrm{n}}}=-\frac{2 \mathrm{R} \mathrm{ch} \mathrm{Z}^{2}}{\mathrm{n}^{2}}, \mathrm{U} \propto \frac{\mathrm{Z}^{2}}{\mathrm{n}^{2}}\)
(c) Total energy
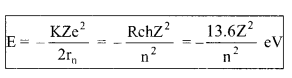
E ∝ \(\frac{Z^{2}}{n^{2}}\)
(d) Binding Energy B.E. = \(\frac{13.6}{n^{2}}\) Z2 eV
BE ∝\(\frac{Z^{2}}{n^{2}}\)
6. Energy, frequency and wave number of emitted or abosrbed photons by an atom
(a) Energy of photon
h╬╜ = (En2 – En1)
= \(\frac{2 \pi^{2} \mathrm{K}^{2} \mathrm{me}^{4}}{\mathrm{h}^{2}}\left[\frac{1}{\mathrm{n}_{1}^{2}}-\frac{1}{\mathrm{n}_{2}^{2}}\right]\)
(b) Frequency of photon
\(\bar{v}=\frac{2 \pi^{2} K^{2} m e^{4}}{h^{3}}\left[\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right]\)
(c) Wave number
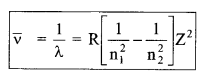
Z = 1 for H-atom
Where R is Rydberg constant
R = \(\frac{2 \pi^{2} K^{2} m e^{4}}{c h^{3}}\) = 1.1 × 107 m-1
For a Bohr atom of atomic number
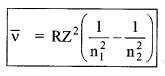
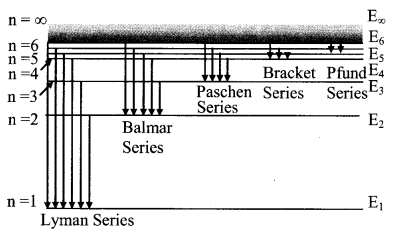
7. Various series of hydrogen spectrum
(i) Lyman series (in ultra violet region)
\(\bar{v}=\frac{1}{\lambda}=R\left[\frac{1}{1^{2}}-\frac{1}{n_{2}^{2}}\right]\), n2 = 2, 3, 4……….
Minimum wavelength λmin = 912 Å
(ii) Balmar Series (In visible region)
\(\vec{v}=\frac{1}{\lambda}=R\left[\frac{1}{2^{2}}-\frac{1}{n_{2}^{2}}\right]\), n2 = 3, 4, 5 ………….
Minimum wavelength λmin = 3645 Å
(iii) Paschen series (Infra red region)
\(\bar{v}=\frac{1}{\lambda}=\mathrm{R}\left[\frac{1}{3^{2}}-\frac{1}{\mathrm{n}_{2}^{2}}\right]\), n2 = 4, 5, 6…….
Minimum wavelength λmin = 8210 Å
(iv) Brackett series
n1 = 4 and n2 = 5, 6, 7……….
(v) Pfund series
n1 = 5 and n2 = 6, 7, 8………
(vi) If an electron transition takes place from n-th orbit, then maximum number of lines m the spectrum = \(\frac{n(n-1)}{2}\)
8. Ioniztion and excitation energies, ionization and excitation potentials
Ionization energy = (EΓê₧ – En) eV
Excitation energy = (En+1 – En) eV
Ionization Potential = (EΓê₧ – En) V
Excitation Potential = (En+1 – En) V
9. Quantum numbers
- Principal quantum number (n): possible values of n = 1, 2, 3…….
- Orbital quantum number (l): possible values from 0 to (n – 1).
- Orbital magnetic quantum number (m1): possible values are (2l + 1) integral from -l to +l.
- Spin quantum number (s) – possible values are (+\(\frac{1}{2}\)) and (-\(\frac{1}{2}\))
Orbital angular momentum = \(\frac{h}{2 \pi} \sqrt{l(l+1)}\)
Spin angular momentum = \(\frac{h}{2 \pi} \sqrt{s(s+1)}\)
10. Numbers of electrons
- Maximum number of electrons in an orbit 2n2.
- Maximum number of electrons in a sub-orbit = 2(2l + 1)
11. PauliΓÇÖs exclusion principle
No two electrons in an atom can have the same set of four quantum numbers.
Visit the online portal Physicscalc.Com to understand the concepts of Physics much better and clarify your ambiguities.