Photoelectric Effect and Photon Formulas
The Concept of Photoelectrons is quite crucial and you need to be aware of it to understand similar topics. Have a glance at the Photoelectric Effect and Photon Formulas listed to get a good hold on the concept. Rather than mugging up try to understand the logic behind the concept so that you can remember the Formulae much easier. Photoelectric Effect and Photon Cheat Sheet will cover formulas for Photoelectric current, Einstein's Photoelectric Equation, Laws of Photoelectric Effect, etc. For any kind of guidance regarding the subject Physics, you can look at Physics Formulas and learn accordingly.
Photoelectric Effect and Photon Formulas Sheet
1. Photoelectric effect
When light of particular wavelength or frequency falls on a metal, electrons are emitted from it. This phenomenon of emission of electrons from a metal surface is called photoelectric effect. The electrons emitted are called photoelectrons.
2. Laws of photoelectric effect
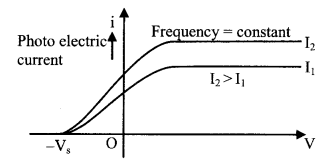
(i) The number of photoelectrons emitted from a metal surface in a unit time (the rate of emission of photoelectrons) is proportional to the intensity of incident light.
(ii) The maximum kinetic energy of the emitted photoelectrons increases with increasing frequency or decreases with increasing wavelength of the incident light. The kinetic energy of the electrons does not depend upon the intensity of the incident light.
(iii) The emission of photoelectrons occurs up to a definite minimum frequency (maximum wavelength) of the incident light. This minimum frequency is called threshold frequency and the maximum wavelength is called threshold wavelength. Threshold wavelength depends upon the nature of the substance and it is different for different metals.
(iv) Within the limits of accuracy (~10-9 second) there is no time lag between the incidence of light at the metal surface and the emission of electrons from the metal surface, whatever is the intensity of incident light.
3. Photoelectric current and stopping potential
(i) The negative potential of the plate relative to the electron emitter, i.e. cathode at which photoelectric current becomes zero is called stopping potential.
It measures the maximum kinetic energy of the photoelectrons.
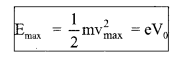
(ii) The stopping potential does not depend upon the intensity of incident light.
4. Work functions
The minimum energy per photon given to the free electrons of the metal which enables them to cross the potential barrier present at the surface of the metal is called work function.
W0 = h╬╜0 = hc/╬╗0
5. EinsteinΓÇÖs photoelectric equation
(a)
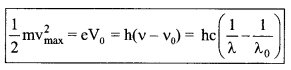
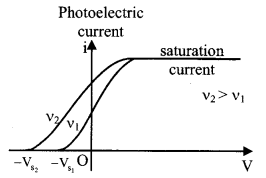
For a given intensity, stopping potential depends on frequency. If v2 > v1, Vs2 > Vs1
(b) Maximum, velocity of emitted electrons
vmax = \(\sqrt{\frac{2 h\left(v-v_{0}\right)}{m}}\)
(c)
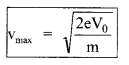
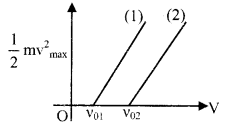
v01 – threshold frequency of metal (1)
v02 – threshold frequency of metal (2)
Looking for any other topic related to the Photoelectric Effect or any random topic of Physics visit Physicscalc.Com and get grip on the relevant concept.