Boyle's Law Calculator
Make the most out of the Boyle's Law Calculator to determine the pressure or volume of a gas easily. Simply enter the needed inputs as per the Boyle's Law Equation and find the unknown parameter in a matter of seconds.
Boyle's Law Definition
Boyle's Law states the relationship between pressure and volume of a gas at constant temperature and mass. As per it pressure is inversely proportional to Volume. We can put it in other words, i.e. product of pressure and volume of a gas in a closed system remains constant as long as the temperature is constant.
Boyle's Law Formula
Boyle's Law Equation can be stated in the following way i.e. pΓéü * VΓéü = pΓéé * VΓéé where pΓéü and VΓéü are the initial pressure and pΓéé and VΓéé are the final values of gas parameters. Based on the parameter you need to estimate we can rewrite the equation of boyle's law and find out the unknown value easily. Changing the Volume of Gas under Isothermal Conditions we can determine the resulting pressure.
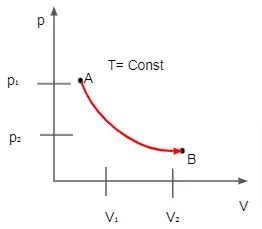
Then Boyle's Equation can be written as pΓéé = pΓéü * VΓéü / VΓéé or pΓéé / pΓéü = VΓéü / VΓéé. From the equation it is clearly evident that ratio of final and initial pressure is inverse of ratio of volumes. We can witness the entire process clearly on a Boyle's Law Graph. Of all those the commonly used type is pressure being a function of volume. And for this process the curve is a hyperbola and this progression works in either ways and both compression and expansion satisfy the Boyle'a Law.
Boyle's Law Example
A fixed amount of a gas occupies a volume of 2L and exerts a pressure of 600 kPa on the walls of its container. What would be the pressure exerted by the gas on being completely transferred to a new container of volume 5 liters. Assume temperature and quantity of gas are constant?
Solution:
Given,
Initial volume (VΓéü) = 2L
Initial pressure (pΓéü) = 600 kPa
Final volume (VΓéé) = 5L
As per BoyleΓÇÖs law, pΓéü * VΓéü = pΓéé * VΓéé
Rewriting the above boyle's law equation to obtain the final pressure exerted by the gas on the walls of the container we have pΓéé = pΓéü * VΓéü / VΓéé
Substituting the known parameters in the above formula we get the equation as such pΓéé = 600 * 2 / 5 = 240 Kpa
Therefore, the gas exterts a pressure of 240Kpa on the walls of the 5 liters container.
Physicscalc.Com has got concepts like friction, acceleration due to gravity, water pressure, gravity, and many more along with their relevant calculators all one under one roof.
Applications of Boyle's Law
Boyle's Law explains all the processes in which temperature remains constant. There are certain areas in which Boyle's Law is applicable and they are explained clearly in the below modules.
- Carnot Heat Engine: It includes 4 thermodynamic processes of which two are isothermal ones and satisfies the Boyle's Law. This method describes the maximum efficiency the heat engine has.
- Breathing: The Process of Breathing can also be linked with Boyle's Law. On taking a breath your diaphragm and intercostal muscles increase the volume of lungs and inturn reduces the gas pressure. When air flows from high presure area to low pressure area air enters the lungs and permits us to take oxygen from environment. In exhalation volume of lungs decreases and thus the pressure inside is higher compared to outside and air flows in opposite direction.
- Syringe: In case of a syringe on pulling the plunger accessible volume increases thus reducing the pressure. As per the Boyle's Law Suction of Fluid Occurs.
Boyle's Law Combined with Charles Law and Gay-Lussac's Law are the fundamental laws that describe several thermodynamic processes.
Frequently Asked Questions on Boyle's Law Calculator
1. What is meant by Boyle's Law?
Boyle's Law states that pressure exerted by a gas is inversely proportional to the volume under Constant Pressure.
2. What is the Formula for Boyle's Law?
The Formula for Boyle's Law is given by pΓéüVΓéü = pΓééVΓéé
3. Why is Boyle's Law Important?
Boyle's Law is necessary to know how gases behave. It proves that pressure and volume are inversely proportional to each other.
4. How is Boyle's Law Different from Charles Law?
In Case of Boyle's Law both Pressure and Volume of Gas are kept at constant temperature. However, in case of Charles Law Temperature and Volume of Gas are Kept at Constant Pressure.
