Unit and Dimension Formulas
Units and Dimensions is a crucial topic that everyone needs to be aware of. In order to increase your level of understanding of the topic, we have mentioned the Unit and Dimensions Formulas complete list here. Formula Sheet for Unit and Dimensions includes Fundamental Quantities, Absolute Error, Fractional Error, Combination of Errors, etc. Go through the list of Physics Formulas existing and be familiar with the similar kind of concepts.
Important Unit and Dimension Formulae
Value Of Physical Quantity
Value of physical quantity = nu (u is unit and n is numeric)
nu = constant or n1u1 = n2u2 or
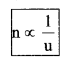
Fundamental Quantities
- Length = meter – L
- Mass = kilogram – M
- Time = second – T
- Electric current = ampere – A
- Temperature = kelvin, ┬░C – K, ╬╕
- Luminous intensity = candella – cd
- Amount of substance = mole – mol
Dimensional Formula
[Ma Lb Tc ╬╕d]
Imp.
1. Always same dimensional quantities can be added or subtracted from each other
2. Measuring system
Conversion
From one system to another system
n2 = n1
Absolute Error
╬öa = Apparent value – Real value
= a = ao
Fractional error and percentage error
Relative error/fractional error =
Percentage error =
Combination of errors
- If x = a + b ⇒ Δx = Δa + Δb
- x = a – b ΓçÆ ╬öx = ╬öa + ╬öb
- x = a/b ⇒
- x = an ⇒
- x =
- x = logea ⇒ Δx =
Common rules for counting signification
Rule 1.
All non zero digits are significant.
Ex. x = 1234 has four significant figures.
Rule 2.
All zero occurring between two non zero digits are significant
Ex. x = 1007 has four significant figure. Again x = 1.0809 has five significant figure
Rule 3.
In a number less than one, all zeros to the right of decimal point and to the left of a non zero digit are NOT significant.
Ex. x = 0.0084 has only two significant digits
Rule 4.
All zeros on the right of the last non zero digit in the decimal part are significant
Ex. x = 0.00800 has three significant figure 8, 0, 0. The zeros before 8 are not significant. Again 1.00 has three significant figures
Rule 5.
All zeros on the right of non zero digit are NOT significant.
Ex. x = 378000 has three significant figures
Rule 6.
All zeros on the right of the last non zero digit become significant, when they come from a measurement.
Ex. x = 3050 m. It has four significant figures.
Arthimetical operations with significant figures
(a) Addition and subtraction: –
In addition or subtraction, the number of decimal places in the result should equal the smallest number of decimal places of terms in the operation.
(b) Multiplication and Division: –
In multiplication and division, the number of significant figures in the product or in the quotients is the same as the smallest number of significant figures in any of the factors.
Ex.
As 9500 has minimum number of significant figures (i.e. 2), therefore, the quotient can have only two significant digits. On rounding off. we obtain the quotient = 930
Some of the non si units in common use are
(a) For length/distance
| 1. Astronomical unit | 1 AU= 1.496 × 1011 m |
| 2. Light year | 1 ly = 9.64 × 1015 m |
| 3. Parallactic second | 1 pc = 3.084 × 1016 m = 3.26 ly |
| 4. Micron or micrometer | 1 ╬╝m = 10-6 m |
| 5. Nanometer | 1 nm = 10-9 m |
| 6. Angstrom unit | 1 Å = 10-10 m |
| 7. X – ray unit | 1 xu = 10-13 m |
| 8. Fermi | 1 f = 10-15 m |
| 9. Yard | 1 yd = 0.9144 m |
| 10. Foot | 1 ft = 0.3048 m |
| 11. Inch | 1 in = 0.0254 m |
| 12. Mile | 1 Mile = 1609.344 m = 1.61 km |
(b) For mass
| 1. Pound | 1 Ib = 0.4536 kg |
| 2. Slug | 1 slug = 14.59 kg |
| 3. Quintal | 1 q= 100 kg |
| 4. Metric tone | 1 t = 1000kg |
| 5. Atomic mass unit | 1 amu = lu = 1.66 × 10-27 kg |
(c) For time
| 1. Minute | 1 min = 60 s |
| 2. Hour | 1 h = 60 × 60 s |
| 3. Day | 1 day = 24 h= 86400 s |
| 4. Year | 1 yr = 365.25 days = 3.156 x 107 s |
| 5. Shake | 1 shake = 10-8 s |
(d) For other quantities
- Litre (for volume) – 1l = 103 cc = 10-3 m3
where cc represents cubic centimeter i.e. cm3. - Gallon (for volume) – In U.S.A., 1 gallon = 3.7854 l
- Pascal (for pressure) – 1 Pa = 1 Nm-2
Pressure exerted by – 1 atm= 1.01 x 105 Pa
earthΓÇÖs atmosphere - Bar (for pressure) – 1 bar = 1 atm. = 1.01 x 105 Pa
- Torr (for pressure) – 1 torr = 1 mm of Hg. col. = 133.3 Pa
- Electron volt (for energy/work) – 1 eV = 1.6 x 10-19 J
- Erg (for energy/ work) – 1 erg = 10-7 J
- Kilowatt hour (for energy) – 1 kWh = 3.6 x 106 J
- Horse Power (for power) – 1 hp = 746 W Γëê
- Diopter (for power of a lens) – 1 D = 1 m-1
- Degree (for angle) – 1┬░ =
Just take help from the website Physicscalc.Com and access the Physics Formula Sheet & Tables existing to have a clear idea of the related concepts.